Abstract
Introduction: Patients with cancer are prone to develop both thromboembolic and atherothrombotic events. Continued advances in contemporary anti-cancer therapy leads to prolonged cancer-specific survival, thus putting an increasing number of patients at high risk of major adverse cardiovascular events (MACE). Therefore, tools to personalize risk stratification to predict and eventually prevent short- and long-term cardiovascular complications in patients with cancer are an unmet medical need. Here, we aimed to assess the predictive utility of cardiovascular biomarkers in a representative population of oncologic patients.
Methods: The study was conducted in the framework of a single-center prospective observational cohort study (Vienna Cancer & Thrombosis Study - CATS). Cardiovascular biomarkers (lipoprotein(a), NT-proBNP, P-selectin, E-selectin, L-selectin, ICAM-1, VCAM-1, sLOX-1) were explored for their predictive utility towards MACE (i.e. composite of non-fatal myocardial infarction or ischaemic stroke, and cardiovascular death), cardiovascular mortality, and all-cause mortality. Patients were followed for the occurrence of MACE for the maximum predefined prospective follow-up period in CATS of 2 years, whereas a prolonged follow-up period (5 years) for mortality outcomes was feasible due to data availability from the national Austrian death registry. MACE and cardiovascular mortality were analysed by competing-risk regression, accounting for non-cardiovascular death as competing outcome event and adjusting for patient age, sex and smoking status. All-cause mortality was analysed in Cox regression analysis, adjusting for age, sex, cancer stage, and tumour type in multivariable analysis.
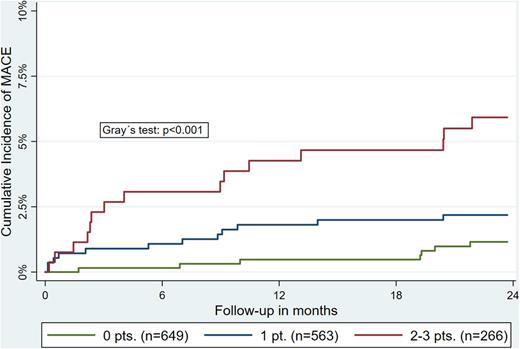
Results: In total, 2,192 adult patients with newly diagnosed or recurrent cancer were included in the present analysis (median age: 62 years; 53% male). Over a median follow-up of 23 months 50 MACEs occurred, with a cumulative 1- and 2-year incidence of 1.7% [95% confidence interval, CI: 1.2-2.3] and 2.4% [95%CI: 1.8-3.1]. An independent association with risk of MACE was observed for baseline levels of NT-proBNP (sub-distribution hazard ratio, SHR, per double increase: 1.28 [95%CI: 1.06-1.54]), ICAM-1 (SHR: 1.53 [1.06-2.20], and L-selectin (SHR: 0.63 [0.44-0.90]), but not for lipoprotein(a), P-selectin, VCAM-1, or sLOX-1. An additive predictive effect of individual biomarkers was observed in the derivation of a point-based risk prediction score (+1 point: NT-proBNP≥75th percentile, ICAM-1≥75th percentile, and L-selectin ≤25th percentile), with a 2-year MACE risk in patents with 0-, 1-, and ≥2 points of 1.5%, 2.2%, and 5.9%, respectively (Figure 1). Furthermore, an independent association with risk of long-term cardiovascular mortality was observed for baseline levels of NT-proBNP (SHR: 1.42 [95%CI: 1.17-1.72]) and ICAM-1 (1.62 [1.05-2.48]). Accordingly, the risk of all-cause mortality independently increased with higher NT-proBNP levels (HR: 1.16 [95%CI: 1.10-1.22]) and ICAM-1 (1.15 [1.06-1.25]). No significant association with cardiovascular mortality or all-cause mortality was observed for levels of lipoprotein(a), P-selectin, E-selectin, L-selectin, VCAM-1, and sLOX-1.
Conclusion: Cancer patients are at substantial risk of cardiovascular events, with a predictive utility of NT-proBNP, L-selectin, and ICAM-1 for short-term MACE-risk. Further, NT-proBNP and ICAM-1 independently predict long-term risk of both cardiovascular mortality and all-cause mortality. Our findings suggest a possible benefit of incorporating cardiovascular biomarkers in personalized risk prediction models to develop individualized cardiovascular prevention strategies in patients with cancer.
Figure 1: Additive effect of biomarkers for the prediction of MACE in patients with cancer. Patients were assigned +1 point each for levels of NT-proBNP ≥75th percentile, ICAM-1 ≥75th percentile, and L-selectin ≤25th percentile. Cumulative incidences were obtained in competing risk analysis, accounting for non-cardiovascular mortality as the competing outcome event.
Disclosures
Liberale:Daiichi-Sankyo: Honoraria. Pabinger:Bayer AG, Boehringer Ingelheim, Daiichi Sanchyo, and BMS/ Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees. Lüscher:Amgen, AstraZeneca, Daiichi-Sankyo, Sanofi, Abbott, Ablative Solutions, Boehringer Ingelheim, Novartis, Servier, and Vifor.: Research Funding; Daichi-Sankyo: Honoraria; DalCor: Honoraria; Novartis: Honoraria; Pfizer: Honoraria. Ay:Bayer, Daiichi Sankyo, BMS/Pfizer, and Sanofi: Honoraria; Bayer, Boehringer Ingelheim, Daiichi Sankyo, and BMS/Pfizer: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal